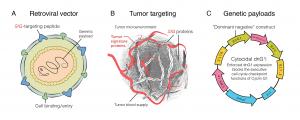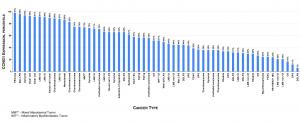

Enhanced CCNG1 expression across all cancer types may predict response to DeltaRex-G, a CCNG1 inhibitor.
USFDA authorizes DeltaRex-G to be used as platform therapy upon which FDA approved cancer drugs may be added for better cancer control (NCT#04091295).
LOS ANGELES, CALIFORNIA, UNITED STATES OF AMERICA, August 3, 2023/EINPresswire.com/ — The Aveni Foundation and the Cancer Center of Southern California/Sarcoma Oncology Center are proud to announce that DeltaRex-G has gained USFDA authorization to expand access to an intermediate size population of advanced breast cancer, in addition to pancreatic cancer, osteosarcoma and soft tissue sarcoma which was originally authorized in 2020. Further, DeltaRex-G may now be given with other FDA approved cancer drugs for these cancer types.
Children as young as 12 years of age with advanced osteosarcoma and soft tissue sarcoma may receive DeltaRex-G with other cancer drugs at pediatric dosing schedules. Unlike chemotherapy, there are few, if any, side effects with this drug.
The human cyclin G1 (CCNG1) is a cancer gene that is found in all tumor types that have been tested to date. DeltaRex-G is a gene-targeted cancer drug with a navigational system that delivers the medicine directly to tumors and carries a CCNG1 inhibitor as its genetic payload to kill cancer cells and their blood supply without collateral damage.
There are long term (>10 years) cancer survivors with DeltaRex-G therapy, giving rise to research on how DeltaRex-G could eradicate cancer stem cells that are responsible for recurrence and the spread of cancer to other organs.
DeltaRex-G is supplied by the Aveni Foundation, an IRS approved 501(c)(3) public charity founded by Dr. Gordon in 2018. Aveni Foundation raises funds through generous donations from benefactors to make the medicine. It is not available through drug or insurance companies.
To donate: Click on the DONATE button at www.avenifoundation.org or send a check to Aveni Foundation, 2811 Wilshire Blvd., Suite 420, Santa Monica CA 90403.
For further information, please visit our websites: www.avenifoundation.org, or contact Dr. Gordon at egordon@avenifoundation.org or egordon@sarcomaoncology.com.
ERLINDA GORDON
Aveni Foundation
+1 818-726-3278
email us here
![]()